Key Takeaways
- Experience has shown that well-designed hybrid courses enhance student learning and increase student retention, even in large introductory science classes.
- Blended learning class guides present digital content in an instructor-guided and consistent format within a course management system.
- Digital learning materials embedded in blended learning guides — interactive multimedia tutorials, podcasts, videos, and simulations — can further engage students, influencing time on task in their learning and promoting their success.
- Use of blended learning class guides in an introductory chemistry course at the Pennsylvania State University's Berks College altered the traditional lecture to a hybrid format and approximately doubled the pass rate for the course.
Blended learning or hybrid courses that combine face-to-face and online learning are increasingly offered at colleges and universities across the United States,1 with growing evidence that they can enhance student learning.2 Their various pedagogies and technologies have prevented acceptance of a single, authoritative model for designing and developing a successful blended course, however. So what can an instructor do? The findings of the U.S. Department of Education's report on evidence-based practices in online learning3 indicate that a significant factor for improving student success is to design courses that increase students' time on task.
This article examines a multiyear pilot project at the Pennsylvania State University's Berks College that redesigned a lecture-based introductory chemistry course into a blended learning course. A central design feature was the creation of blended learning class guides that were integrated into Penn State's course management system, ANGEL. These inventive class guides introduced course content prior to student arrival in the classroom and incorporated various interactive learning materials to increase student engagement with course content. The guides directed students' learning and facilitated their use of the course textbook, assignments, and resources. The online guides also allowed students to spend more time on task outside the classroom. They played a vital role in dramatically altering the traditional lecture format course, leading to increased student success and course retention.4
Hybrid Course Design
During the spring semester of 2005, several key college administrators began considering how to best enhance the learning environment at Penn State Berks. The college's chief information officer proposed creating a course that would enhance the student learning experience by using a blended learning course design. The CIO and the associate dean of Academic Affairs examined all the courses taught at the college to identify those introductory courses with high enrollment and particularly low success rates. One such course was Chemical Principles, an introductory course with five sections and enrollment for each ranging from 60–75 students. Chemical Principles had a low retention rate and was one of the most commonly failed courses.
The college administration wanted to create a blended course that would enhance the students' learning in a measurable way (for example, increase overall course GPA). Also, the course redesign should increase student satisfaction and retention as well as reduce the overall cost of the course. Additionally, the administration hoped that aspects of this hybrid course design could be integrated into other appropriate courses across the curriculum.
At the beginning of the fall 2005 semester, the administration approached two instructors (a tenured associate professor and a tenure-track assistant professor) for the Chemical Principles course and invited them to participate in a hybrid course redesign. Shortly thereafter, a design team was formed consisting of the two faculty members; the director of the Center for Learning and Teaching (CLT), who served as project manager; and an instructional designer from the CLT. Later a newly created position (multimedia specialist) was added, also from the CLT, as well as the director of Planning, Research, and Assessment. This blended learning course redesign team would spend nearly 1,000 hours redesigning the Chemical Principles course at Penn State Berks. The team spent the majority of the first year designing and developing the course, with a second year spent implementing and assessing it.
The course redesign team applied a systematic approach to instructional development of the hybrid course that included learner and course goals/objectives analysis, design and development of learning activities, formative/summative student assessment, and qualitative and quantitative student course performance and satisfaction evaluation.5 The learning analysis phase of the project identified several factors (such as students' recall and remediation of important prerequisite course content and students' preparedness) that the team determined would need to be addressed in order to enhance students' course performance. Consequently, the team focused on facilitating student engagement with the course content both inside and outside the classroom. Past research has established that active learning assignments positively influence students' perception of course value and course engagement.6 The team believed that increasing student engagement would influence their time on task and in turn enhance learning (course test scores and GPA) and lead to an increase in student course retention.7
The classroom design focused on integrating a student response system (clickers) into the course.8 Students were placed into stable peer-led groups.9 This allowed them to work collaboratively on chemistry problems that increased in difficulty, consistent with Bloom's Taxonomy, over the course of a class period. Students' use of the clickers to answer the problems allowed each instructor to tailor the class session based on student needs.10 This dynamic facilitated the use of brief impromptu discussions and lectures when students struggled with a problem. Conversely, the instructor could move on to new topics when students successfully solved problems in the class. Peer mentors are available to assist the students with chemistry concepts and with group interactions as they solve problems in their base groups.
This clicker-focused classroom design allowed the online portion of the class to focus on increasing student engagement with course content prior to and after each class session. Consequently, student time on task was identified as an important component to meeting the goals of the college's administration (increased student learning, satisfaction, and retention).11 The redesign team decided to migrate lecture content, assignments, and quizzes to Penn State's ANGEL CMS so that students could access course content easily and conveniently outside the classroom both before and after class.
During the design phase of this project, however, the redesign team discovered the pedagogical limitations of ANGEL.12 The team realized that moving a vast amount of content (most of the lecture content, assignments, and course quizzes) to ANGEL could cause problems for students, who would probably struggle to navigate the "Lessons" folder structure. The team brainstormed various technology-related solutions and settled on the creation of an interactive, organized, online study tool — the blended learning class guides. These innovative guides were meant to become an essential link between the students' in-class and outside of class learning experiences, moving beyond the traditional class syllabus or online study guide.13 The creation of these class guides would prove invaluable to the design team's ability to implement the desired hybrid course format and pedagogy.
Determining Content of the Blended Learning Class Guide
The class guides were designed to create a highly functional student resource that would clearly identify, scaffold, and organize critical content (foundational knowledge) that students must know to successfully move on to subsequent content.14 Foundational knowledge is specific, and the questions asked by instructors have well-defined and correct answers. Moving foundational knowledge online gave instructors more in-class time available to teach non-foundational knowledge face-to-face.15 Non-foundational knowledge is less specific, and the questions asked might have more than one correct answer. An example of non-foundational thinking involves tap water: one student could argue that tap water is a solution based on the ions present in it, while another student could assert that it is a pure substance made up only of water molecules. Both students could be correct as long as they base their answer on logical reasoning.
As mentioned, too many students were coming to class unprepared. One important function of these class guides was to serve as pre-class study guides that would lead students through a series of key points, activities, and graded assignments (which helped motivate their use of the resource) designed to help better prepare them for each class session. Consequently, the redesign team also agreed that it was appropriate to align the course textbook (Chemistry: The Central Science, used by the entire Chemistry Department at Berks) with much of the content contained in the online guides. Because much of that content was foundational, the group was not concerned that new editions of the textbook would require significant revisions to the guides once created.
The class guides contain content that highlights critical information in each of the textbook chapters as well as a variety of supplementary materials to help students decipher difficult concepts in the book. These include key points, brief notes, summaries, examples, and identification of both important material and material the instructors determined could safely be ignored. For particularly difficult concepts, the guides also provide exercises. The guides avoid excess verbiage by directing students to specific parts of the book. The expectation was that students would more fully use the resources available in the textbook if synergy existed between the class guides and the book.
Students are expected to complete an assignment in the class guide prior to each class. The instructors can thus determine who is spending time on the guides and remind those who are not that completion of the class guide is required. Additionally, ANGEL allows instructors to verify how much time each student has spent on a specific task, enabling them to intervene where necessary.
During the first phase of the hybrid course project, the team reviewed and identified the course goals and objectives, then further broke them down into specific student learning outcomes for each topic/chapter of content covered throughout the entire course. To help guide the learners to a clear understanding of what they should be able to do once they had completed the course, the team decided that students needed to have plainly and explicitly stated learning objectives for each chapter of the textbook that corresponded to the appropriate class session.
To make the guides student-centered and provide an activity-based learning environment, each class guide included both textbook chapter practice exercises and an instructor-created pre-class assignment. While the textbook chapter practice exercises were not graded, the pre-class assignments were. The pre-class assignments aimed to facilitate student learning by motivating them through low-stakes graded assignments that engaged them in understanding, applying, analyzing, or evaluating course content immediately prior to the class session that would address that same content.16
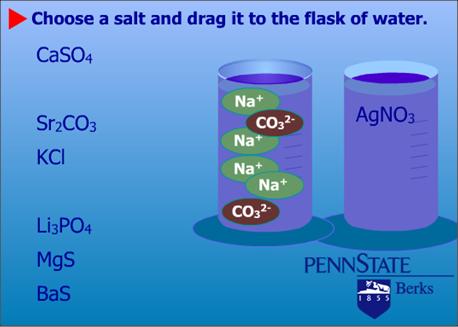
In addition to the materials that assist students in using the textbook effectively, the blended learning class guides make extensive use of multimedia resources such as instructor podcasts, animations, short videos, modules (tutorials with practice exercises), and simulations.17 To determine what types of multimedia resources to include in the class guides, the team analyzed several years' worth of test scores to look for topics or concepts that a majority of students tended to struggle with consistently (for example, balancing chemical equations; see Figure 1).
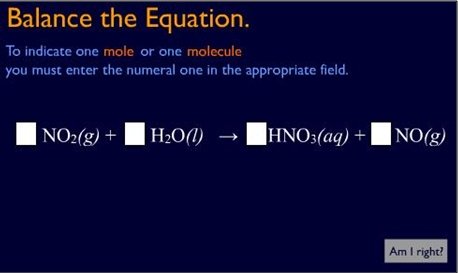
Figure 1. Balancing Equations — Interactive Practice
After the team identified the troublesome topics, they reviewed the related multimedia resources provided with the textbook. Only those resources (animations, video clips) that the instructors approved were integrated and embedded into the "Learning Resources" section of the guides. Because the course instructors did not select the majority of textbook-related content, the team decided to search for freely available existing multimedia interactive learning materials. Very few of the freely available resources located met with the instructors' approval. Consequently, the team decided to develop additional multimedia (mini-podcasts three to five minutes in length) and interactive resources (such as Salts and Ions — Interactive Practice) in-house to further enhance the students' engagement and understanding of the identified course content (see Figure 2).
Figure 2. Additional Multimedia Learning Resource
Constructing the Blended Learning Class Guides
The redesign team placed a great deal of emphasis on the design and development of the course guides because these guides were essential in freeing up class time and allowing the move from a traditional lecture-based format to a peer-led team-based approach in the classroom. The class guides were constructed through an iterative process, with subsets of the redesign team working on specific class guide creation tasks, and then all the team members reviewing and signing off on each class guide.18 The six primary components of the guides (see Figure 3) were handled by different members of the team depending on their expertise and role within the redesign. (See "Workflow" for individual assignments.)
| Simple "How To" directions |  |
| Interactive 'clickable' Table of contents |  |
| Clearly stated student learning objectives |  |
| Action Items
|  |
| Multimedia Learning Resources (i.e. tutorials, practice simulations, podcasts, animations, and videos) |  |
| Printable (text-only) version of the Class Guide |  |
Figure 3. Six Key Components of the Blended Learning Class Guides
Figure 4. CHEM 110 Class Guide
Underlying Technology
One of the first challenges the team faced in creating the online guides was determining the most appropriate technology to use. The principal criteria applied to the selection of technology were: cost and development time, ease of maintaining and updating for faculty, and ease of use for students. The team decided that using a combination of mature and simple to use technologies was the most sensible choice, which led to initially designing and developing the guides in Microsoft PowerPoint. Because of a university-wide licensing agreement, PowerPoint was freely available software that both faculty members were comfortable using. Also, it was possible to use this software to create a pleasing design background, develop navigation buttons, and integrate hyperlinks. This PowerPoint class guide would then be saved in a Portable Document Format (PDF) before being uploaded to ANGEL. This would allow the instructor to upload a self-contained guide that could not be edited by the students but allowed universal access through a freely available Adobe Reader software package preinstalled on all campus lab computers as well as being downloadable for the students' personal computers.
Navigation & Organization
The redesign team wanted to create a simple to use, intuitive navigation system. Additionally, the team decided that the navigation system should facilitate both linear and nonlinear navigation by students. Consequently, directional arrow buttons were added to each of the class guide's slides to allow students to go forward, back, and return to a Table of Contents page (see Figure 5). This linear structure enables the instructor to guide students based on the faculty member's desired presentation order of the class guide content. However, to allow students to quickly jump to a specific section of content for review, the guides needed to allow nonlinear navigation as well. The team decided that a Table of Contents page with active hypertext links on the contents would facilitate this type of self-guided study.

Figure 5. Class Guide Table of Contents
The guides were organized so that they would cover the topics appropriate for each week of class. The six components were sequentially ordered so that each guide included a "How To" use the class guides page, followed by a table of contents, specific topic/chapter learning objectives, action items for the subsequent class session, a learning resources page, and lastly a printable text-only version of the guide (see Figure 3).
Delivery
The course guides were integrated directly into the chemistry course site housed in the ANGEL CMS. Students can easily access the appropriate course guide by simply clicking on the corresponding topical class session folder and then on the displayed icon that loads the guide as an Adobe Acrobat .pdf file onto the student's computer. The advantage of integrating the guides into ANGEL was that it allowed the design team to place all of the course content (excluding the full text of the textbook) into the CMS. A significant benefit to this placement is that it allows the faculty to see when students are accessing the guides and, therefore, if they are coming prepared for class.
Overall Hybrid Course Design Results
The preliminary quantitative analysis of the impact of the redesigned course indicates that the hybrid course has enhanced student learning as measured by both the greater number of students who now receive a passing grade (C or better) and an overall increase in student class grade point average in the course. Data were collected from a variety of sources including student grades, number of withdrawals, success in general chemistry (students receiving a grade of C or better), final exam grades, and student evaluations of teaching effectiveness. When the hybrid course was implemented, the redesign team decided that all sections would be taught using the new methodology. Therefore, students enrolled in the non-hybrid course from the prior five years (fall 2001 through spring 2006) were used as a control group. To ensure that the students held to a normal distribution — values hold to a bell-shaped curve and have a variance of one — measures of skewness and kurtosis were run using SPSS 17.0. The data were considered skewed (the curve was not bell-shaped), thus Kruskal-Wallis analyses were run. Kruskal-Wallis calculations are similar to analysis of variance (ANOVA) calculations but do not rely on normalized data. If the Kruskal-Wallis asymptotic significance (AS) value is less than or equal to 0.05, the means of the populations can be considered significantly different.
The team suspected that the fall semester students differed academically from their spring or summer counterparts. An analysis found that the three groups differed in their quantitative, verbal, and total SAT scores (AS = 0.000), so the students were separated into groups. The summer students were then excluded from the analysis because the sample size was comparatively small. The remaining students were placed into one of four groups:
- Non-hybrid fall students (883 participants)
- Non-hybrid spring students (665 participants)
- Hybrid fall students (177 participants
- Hybrid spring students (273 participants)
The hybrid students participated from the fall 2006 through spring 2008 academic years.
When looking at a distribution of course grades, the most common grade earned by the non-hybrid fall students was a W (withdrawal), while the fall hybrid students earned a C. The spring non-hybrid students and the spring hybrid students most commonly earned a C. Although the spring students' grades were the same, changing the course format significantly helped those students who took the course in the fall semesters. Another metric used to determine the success of the hybrid course was the ratio of students who passed (earned an A, B, or C, inclusive of + and -) versus those who failed (earned a D, F, or W). This means that a higher ratio should indicate a greater number of students successfully passing the course. The C cutoff was used because most majors at the university require a grade of C or better in the chemistry course. Only 1.3 fall non-hybrid students passed the traditional lecture course for every 1 student who failed. After the course was redesigned, 2.1 fall hybrid students passed for every 1 student who failed. The difference between these was significant (AS = 0.000). The spring non-hybrid students also passed at a much lower rate than their hybrid counterparts, with 1.8 spring non-hybrid students passing the course for every 1 who failed, while 2.3 hybrid spring students passed for every 1 who failed (AS = 0.022). Changing the course from a lecture format to a hybrid course led to approximately twice the number of students passing the course.
To compare student grades in the hybrid and non-hybrid courses, letter grades were converted to numeric grades using the scale at Penn State University (see Table 1). By using an independent samples z-test, the team calculated that the hybrid students in the fall semester earned a significantly higher GPA (1.97) than their non-hybrid fall counterparts (1.66, p = 0.003). The significantly higher GPA continued in the spring semesters, with the hybrid spring students earning a 2.05 and their non-hybrid spring counterparts earning a 1.85 (p = 0.035), as shown in Figure 6. The technological enhancements in the redesigned hybrid course raised the students' course grades to almost passing in the fall semesters and to passing grades in the spring semesters.
Table 1. GPA Scale Equivalents of Letter Grades
| Letter Grade | A | A- | B+ | B | B- | C+ | C | D | F | W |
| GPA Equivalent | 4.00 | 3.67 | 3.33 | 3.00 | 2.67 | 2.33 | 2.00 | 1.00 | 0.00 | 0.00 |
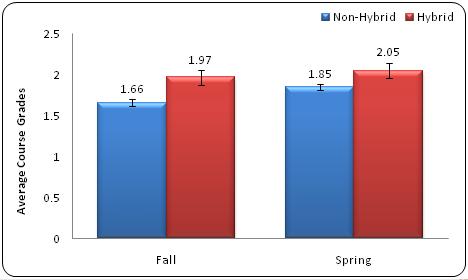
Figure 6. Average GPA in General Chemistry
To determine the effect of the class guides on student outcomes in the course, the amount of time spent on the class guides in the fall semester of 2008 was compared to individual student grades on the final exam. The students were split into four groups:
- Those who spent less than two hours on the class guides
- Those who spent between two and four hours on the class guides
- Those who spent between four and six hours on the class guides
- Those who spent more than six hours on the class guides
Students who spent more time on the class guides had a higher grade on the final exam. Interestingly, those students who spent more than six hours on the class guides had a lower grade on the final that those who spent between four to six hours. These students were possibly distracted or multitasking while using the class guides, but it is also possible that they were looking for more help than the class guides offered and would have been better served seeking additional help from an instructor. Even taking this dip of grades into account, the more time the students spent on the class guides led to a significantly higher grade on the final exam (AS = 0.012), as shown in Figure 7.
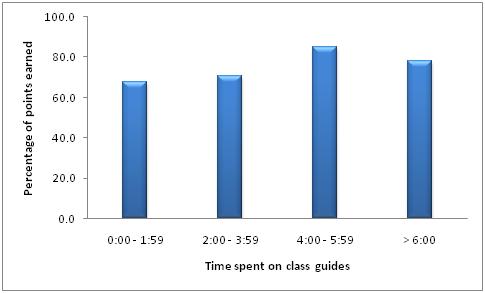
Figure 7. Time Spent on the Class Guides Versus Grade on the Final Exam
Assessment procedures in the lecture and hybrid courses varied, as different instructors had homework, quizzes, class participation, or some combination thereof in their grading schema. To ensure that any increase in student success after hybridization did not come solely from an increase in available points, such as clicker or pre-class assignment points, the final exam given during the spring semester of 2006 (the last semester the course was taught as a lecture) was given in the spring semester of 2008. (The finals in 2006 were never returned to the students.) The average grade on the final during the lecture section was 58 (±3) out of 100 points, while in 2008 it increased to 70 (±2) (p = 0.025).
The team also developed a course survey questionnaire for students to fill out at the end of each semester. This survey included open-ended questions that asked students to list tools that were helpful to them when they were trying to learn the concepts in the course. The open-ended nature of the question meant students selected those parts of the course that helped them without prompting. Not surprisingly, many students listed several course components, so the following percentages do not add to 100 percent. Over 40 percent of the respondents listed online quizzes as helpful, and 35 percent listed the class guides. The use of clickers was noted by over 40 percent of the respondents, which indirectly relates to the preparation students accomplished using the class guides outside of and prior to each class session in order to successfully answer the clicker questions. Table 2 shows the results of the student survey.
Table 2. Survey Results
| Item Listed by Student as Being Helpful to Success | Percent of Students Listing Item |
| Group work | 46.0% |
| In class work/clickers | 41.5% |
| Online quizzes | 40.2% |
| Class guides | 35.1% |
| Instructor/mentor | 13.8% |
| Text | 11.5% |
| Exams | 4.6% |
Many students initially struggled with the radically different teaching and learning environment this hybrid course presented. The students complained, especially the first semester, that the instructor was no longer "teaching" them anything. However, the culture on campus has quickly changed. Now, students entering the course have embraced the new style of teaching and are often disappointed when it is not used in subsequent courses. As evidence of this, over 93 percent of student respondents either strongly or somewhat agreed that the blended course design improved their learning.
Finally, student evaluations of the courses were compared. The two evaluation measures analyzed were "rate the overall quality of the course" and "rate the overall quality of the instructor." These two questions are used by the administration to rate the quality of a faculty member's teaching (and thus correspond directly to faculty raises and possible tenure). The evaluations are anonymous, not available to the instructors until the following semester, and on a scale of 1 to 7, with 7 being outstanding and 1 being poor. During the fall semesters, the non-hybrid course had an overall weighted average quality score of 4.79, while the hybrid course had a 5.42. The instructors also had a perceived increase in quality, with the weighted average going from 4.76 to 5.89. This trend continued into the spring semesters, with the non-hybrid course having an overall quality of 4.65 while the hybrid course earned a 5.30. The non-hybrid instructors earned an overall quality rating of 4.78, and the hybrid instructors earned a 5.92.
Hybrid Chemistry Course Success: Final Reflections
As a result of the improved student success and retention, the faculty in the chemistry department have committed to teaching the General Chemistry course in the blended format. This has created course consistency among all the sections because the students receive identical materials each class day and take a variation of the same exam. The time spent preparing for the class by individual instructors has also dramatically decreased because the lecture has been replaced with in-class problem-solving activities and the online blended learning class guides. The instructors collaborate to ensure that the problems given to students are appropriate and relevant, but no individual instructor is responsible for the whole course. The college's administration is also pleased with the overall results of the Chemical Principles hybrid course.
The blended learning class guides aided in the transformation of a traditional lecture-based course into a successful hybrid course. The class guides enabled the instructors to move much of the lecture content outside the classroom, and as a result allowed them to create a peer-led team-learning environment in class. The chemistry faculty, through the use of clickers, can more actively guide and engage students in learning in the classroom. A central aim of the guides was to expose the students to important content just prior to the class session covering the material, enabling them to come to class better prepared. The guides encouraged student use in a number of ways, including assigning a few points to the pre-class assignment component, providing a helpful textbook study guide resource, and integrating both multimedia and interactive learning materials that can engage various student learning styles.
The chemistry faculty who have taught both lecture and hybrid formats have noticed an increase in student preparedness. The research evidence from the student course questionnaire, combined with the evidence that the more time students spent using the blended learning class guides, the better they scored on their final exam, supports the supposition that the guides are an integral component in creating this successful hybrid course. However, the team realizes that because the traditional lecture course did not have any means of capturing the amount of time on task students spent outside the classroom, it is impossible to directly calculate and compare the impact of the guides on the blended learning format versus the lecture. Additionally, the redesign team realizes that other factors in the Chemical Principles hybrid course redesign also contributed to student success and retention. In the future, the team plans to examine the extent to which the use of clickers, multimedia, and online quizzes contributed to enhancing student success in the course.
The blended learning class guides provide more than a detailed syllabus or simple online study guide. The integration of instructor-guided materials, practice exercises, and interactive, multimedia tutorials, games, and simulations in the documented guides format has shown promise in assisting lower level, general education chemistry students in better understanding course content, which has led to improved student test scores and course retention. While not every aspect of a class guide may be appropriate for all hybrid courses, this type of guide could be replicated, customized, and utilized in a variety of hybrid or online course learning environments to further enhance student achievement and retention.
- D. Randy Garrison and Normal D. Vaughan, Blended Learning in Higher Education: Framework, Principles, and Guidelines (San Francisco: Jossey-Bass, 2008).
- Barbara Means, Yukie Toyama, Robert Murphy, Marianne Bakia, and Karla Jones, "Evaluation of Evidence-based Practices in Online Learning: A Meta-Analysis and Review of Online Learning Studies," U.S. Department of Education, Washington, D.C., 2009.
- Ibid.
- Wilfried Admiraal, Theo Wubbels, and Alfred Pilot, "College Teaching in Legal Education: Teaching Method, Students' Time-on-Task, and Achievement," Research in Higher Education, vol. 40, no. 6 (1999), pp. 687–704.
- Walter Dick, Lou Carey, and James O. Carey, The Systematic Design of Instruction, Sixth Ed. (Allyn & Bacon, 2004).
- Kevin S. Floyd, Susan J. Harrington, and Julie Santiago, "Improving I.S. Student Engagement," Proceedings of the 12th Annual Conference of the Southern Association for Information Systems (SAIS), Charleston, SC, March 13, 2009, pp. 24–29.
- Alberto F. Cabrera, Amaury Nora, and M. B. Castaneda, "College Persistence: Structural Equations Modeling Test of an Integrated Model of Student Retention," Journal of Higher Education, vol. 64, no. 2 (March/April 1993), pp. 123–139.
- Kirsten Crossgrove and Kristen L. Curran, "Using Clickers in Nonmajors- and Majors-Level Biology Courses: Student Opinion, Learning, and Long-Term Retention of Course Material," CBE Life Sciences Education, vol. 7, no. 1 (Spring 2008), pp. 146–154.
- Leo Gafney and Pratibha Varma-Nelson, "Evaluating Peer-Led Team Learning: A Study of Long-Term Effects on Former Workshop Peer Leaders," Journal of Chemical Education, vol. 84, no. 3 (March 2007), pp. 535–39; David K. Gosser and Vicki Roth, "The Workshop Chemistry Project: Peer-Led Team-Learning," Journal of Chemical Education, vol. 75, no. 2 (February 1998), pp. 185–187; Carl C. Wamser, "Peer-Led Team Learning in Organic Chemistry: Effects on Student Performance, Success, and Persistence in the Course," Journal of Chemical Education, vol. 83, no. 10 (October 2006), pp. 1562–1566; and Kenneth S. Lyle and William R. Robinson, "A Statistical Evaluation: Peer-led Team Learning in an Organic Chemistry Course," Journal of Chemical Education, vol. 80, no. 2 (February 2003), pp. 132–134.
- Lorin W. Anderson and David R. Krathwohl, eds., A Taxonomy for Learning, Teaching, and Assessing: A Revision of Bloom's Taxonomy of Educational Objectives, Complete Edition (New York: Longman, 2001).
- Arthur W. Chickering and Stephen C. Ehrmann, "Implementing the Seven Principles: Technology as Lever," AAHE Bulletin, (October 1996), pp. 3–6.
- Lisa M. Lane, "Toolbox or Trap? Course Management Systems and Pedagogy," EDUCAUSE Quarterly, vol. 31, no. 2 (April–May 2008), pp. 4–6.; and Jonathan Mott, "Envisioning the Post-LMS Era: The Open Learning Network," EDUCAUSE Quarterly, vol. 33, no. 1 (January–March 2010).
- Alison Carr-Chellman and Philip Duchastel, "The Ideal Online Course," British Journal of Educational Technology, vol. 31, no. 3 (July 2000), pp. 229–241.
- Anne Jelfs, Roberta Nathan, and Clive Barrett, "Scaffolding Students: Suggestions on How to Equip Students with the Necessary Study Skills for Studying in a Blended Learning Environment," Journal of Educational Media (now Journal of Learning, Media, and Technology), vol. 29, no. 2 (July 2004), pp. 85–96.
- Kenneth A. Bruffee, Collaborative Learning: Higher Education, Interdependence, and the Authority of Knowledge, 2nd ed. (Baltimore, MD: John Hopkins University Press, 1999).
- Robert M. Gagne and Walter Dick, "Instructional Psychology," Annual Review of Psychology, vol. 34, no. 1 (February 1983), pp. 261–295.
- A. Picciano, "Blending with Purpose: The Multimodal Model," Journal of the Research Center for Educational Technology, vol. 5, no. 1 (2009), pp. 4–14.
- Emily Hixon, "Team-Based Online Course Development: A Case Study of Collaboration Models," Online Journal of Distance Learning Administration, vol. 6, no. 4 (Winter 2008); and Badrul H. Khan, "The People-Process-Product Continuum in E-Learning: The E-Learning P3 Model," Educational Technology, vol. 44, no. 5 (September/October 2004), pp. 33–40.
© Katie E. Amaral and John D. Shank. The text of this article is licensed under the Creative Commons Attribution-Noncommercial-No Derivative Works 3.0 license.